Ionic Bonds
Salts
In nature there are many different minerals we call "Salts". If you look very closely at the particles of table salt, you will find little cubic crystals. These salt crystals are made of sodium chloride (NaCl), and their cubic shape is due to a very specific arrangement of the atoms. As discussed earlier, all of the minerals classified as salts are held together through ionic bonds. The arrangement of atoms, the shapes of the crystals they form, and their mechanical, thermal, and electrical properties are all due to this special type of bonding.
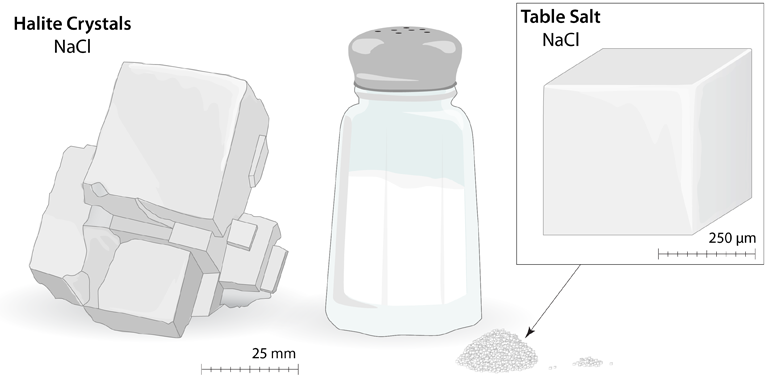
Table Salt: Large cubic crystals of sodium chloride (NaCl) are often found in nature (Halite); this is the same material that makes up the tiny crystals of table salt. The cubic arrangement of atoms, and the cubes that are visible in these large Halite crystals and the small grains of table salt, is called the rock salt structure. These cubes are the result of a very specific arrangement of the ions.
Rock Salt
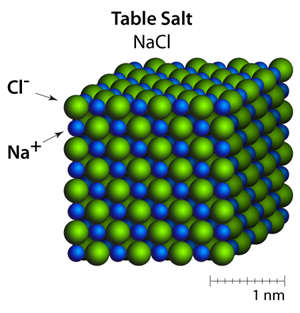
The cubic shape of the Halite crystals comes from the organization of the sodium (Na+) and chloride (Cl–) ions within the crystals — this is often called the rock salt structure. In this particular structure the shape that you see (the crystal habit) is the same shape as the crystal structure.
Ionic solids are a group of materials that are held together through ionic bonding. The packing configuration is a result of both the attraction between opposite charges and the size of the ions. For example, the positive ions are strongly attracted to the negative ions, and are trying to surround themselves with as many ions of the opposite charge as possible. However, the negatively charged ions are also strongly repelling from one another and are trying to surround themselves with positively charged ions. In the 3 dimensional case of Halite, this balance of attraction and repulsion results in a very stable and structured arrangement of ions where each ion has 6 immediate neighbors of opposite charge.
In this molecular dynamics simulation framework you can create 4 different model ionic solids: sodium chloride (NaCl), calcium chloride (CaCl2), sodium sulfide (Na2S), and calcium sulfide (CaS) — notice that these materials are charged balanced (if they weren't then the electrostatic forces would be so great that it would literally tear them apart!).
| charge | number | charge balance | ||||||
| anion(1) | cation(2) | z1 | z2 | n1 | n2 | n1 z1 + n2 z2 = 0 | ||
| Sodium Chloride | (NaCl) | Na+ | Cl– | +1 | –1 | 1 | 1 | 1 · 1 + 1 · (–1) = 0 |
| Calcium Chloride | (CaCl2) | Ca2+ | Cl– | +2 | –1 | 1 | 2 | 1 · 2 + 2 · (–1) = 0 |
| Sodium Sulfide | (Na2S) | Na+ | S2– | +1 | –2 | 2 | 1 | 2 · 1 + 1 · (–2) = 0 |
| Calcium Sulfide | (CaS) | Ca2+ | S2– | +2 | –2 | 1 | 1 | 1 · 2 + 1 · (–2) = 0 |
Recall that the Coulombic forces are proportional to the product of the charges. Thus, at the same spacing the forces between the ions in calcium sulfide (Ca2+ & S2–) are four times greater (4x) than the forces between the ions in sodium chloride (Na+ & Cl–). The crystallographic spacing of these two ionic solids is actually very similar, but the difference in the bond strength is significant — notice the trends between the melting points and the ionic charges.
| charge | charge product |
||||||
| anion(1) | cation(2) | z1 | z2 | –(z1.z2) | melting point | ||
| Sodium Chloride | (NaCl) | Na+ | Cl– | +1 | –1 | 1 | 801 °C |
| Calcium Chloride | (CaCl2) | Ca2+ | Cl– | +2 | –1 | 2 | 772 °C |
| Sodium Sulfide | (Na2S) | Na+ | S2– | +1 | –2 | 2 | 1,176 °C |
| Calcium Sulfide | (CaS) | Ca2+ | S2– | +2 | –2 | 4 | 2,525 °C |
Experiments (can be loaded directly into Salts app)
|
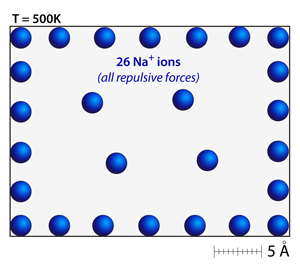
Try adding a number of ions that are all the same type or have the same charge. Notice that the ions distribute themselves throughout the simulation and attempt to maximize the spacing between one another; like charges repel. |
|
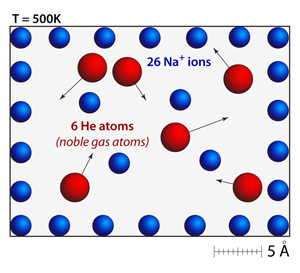
The addition of a neutral atom (helium) has essentially no effect on the spacing or motion of the ions. Even though the helium atoms are bouncing around within the simulation the strength of the repulsion among the ions is so strong that they barely respond to the collisions. Additionally, the helium atoms have significantly less momentum because of their low atomic mass. In this simulation the helium atoms are drawn according to their van der Waals radius, which is why they appear to be so much larger than the sodium ions. |
|
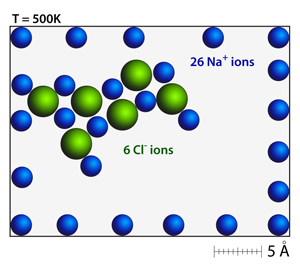
The addition of chloride anions to the sodium cation field will immediately cause the spatial distribution of the cations to distort; it will also begin to produce new crystal structures. Even a small number of anion-cation pairs will begin to form the rock salt structure — look for patterns of alternating positive and negative ions. |
|
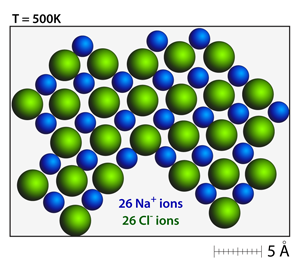
Stable crystals will form when the there is a balance of positive and negative charges. Notice the sharp corners and charge balanced crystal faces in this rock salt structure. |
|
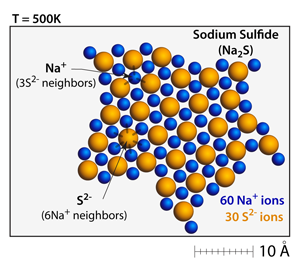
Ionic solids such as sodium sulfide (Na2S) and calcium chloride (CaCl2) can also be made. In these structures charge is balanced through the packing arrangements and the different numbers of ions depending on the charges. |
|
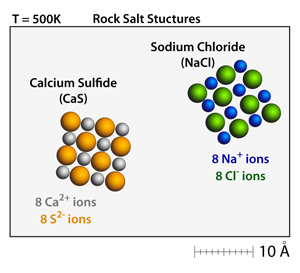
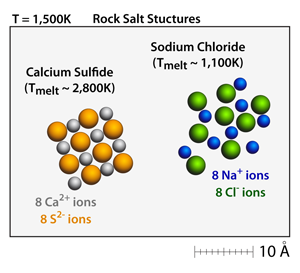
Both calcium sulfide (CaS) and sodium chloride (NaCl) have the rock salt structure. Small crystals can be made and moved throughout the simulation by dragging on the corners of the crystals. At 500K these crystals will be well defined and stable. |
|
Calcium sulfide (CaS) has a +2 –2 relationship between the cations and the anions, respectively. This results in a bond strength that is approximately 4x greater than the corresponding bond strength in sodium chloride (NaCl), which has a +1 –1 relationship between the cations and anions. As a result, the melting point of calcium sulfide (CaS) is over 2x greater than the melting point of sodium chloride (NaCl). |
|
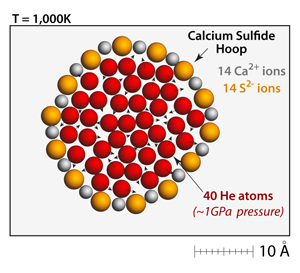
One way to measure the strength of the ionic bonds is to create a closed ring of cations and anions and slowly add helium atoms to the interior. At this temperature and concentration the pressure from the helium is immense; the internal pressure is almost 1 GPa! Eventually, under this pressure, the hoop structure will fail and the helium atoms will escape. |
|
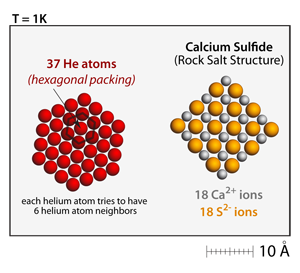
The helium atoms in this simulation are drawn according to their van der Waals radius, which is much larger than the crystal radius used to draw the ions. At very low temperatures (1–3K), the helium atoms will also condense and form solids. Their solidification pattern is a close packed arrangement that has each helium atom being surrounded by 6 other helium atoms. There is also a van der Waals interaction between the helium atoms and the ions. So, at low enough temperatures helium atoms will also condense onto the salt crystals. |
Enjoy the Atoms!